Introduction:
CD19-targeting chimeric antigen receptor (CAR) T cell therapy has generated robust clinical responses in the treatment of relapsed and refractory hematological malignancies. While CAR T cell toxicity in the form of cytokine release syndrome (CRS) is well-characterized, there is limited data describing the potential cardiovascular toxicities following CART cell therapy. The goal of this study was to characterize these cardiovascular toxicities and describe their relationship to CRS.
Methods:
We performed a retrospective analysis of 60 patients who received Axicabtagene ciloleucel (n=43), Tisagenlecleucel (n=16) or investigational bispecific CD19/CD20 CART cells (n=1) at the University of California Los Angeles between 2018 and 2020. The American Society for Transplantation and Cellular Therapy (ASTCT) consensus grading system was used to identify CRS. Cardiovascular toxicities were defined as a composite of newly developed arrhythmias, clinical heart failure, acute coronary syndromes, or any cardiac death. Chi-square, Fisher's Exact test, and t-test analyses were used to identify factors associated with the development of cardiovascular toxicities following CAR T cell infusion.
Results:
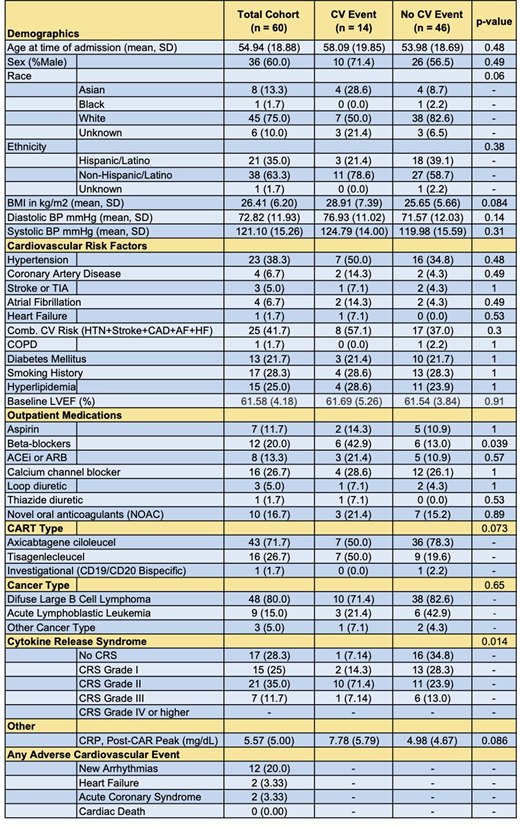
Sixty patients with a mean age on admission of 54.94 (±19) years underwent CAR T cell therapy with a median time to follow-up or death of 206 days (Table 1). Overall, 15 patients developed grade I CRS, 21 patients developed grade II CRS, and 7 patients developed grade III CRS. No patients progressed past grade III CRS. In total, 14 patients (23.3%) developed adverse cardiovascular outcomes, including arrhythmias (n=10), clinical signs of heart failure (n=2), and acute coronary syndrome (n=2). In a 2-sided Fisher's Exact Test, the grade of CRS developed was significantly associated with the development of adverse cardiovascular outcomes in our patient cohort (p=0.014). Those developing cardiovascular events were also more likely to be taking beta blockers prior to the initiation of CAR T cell therapy (p=0.039). There was however no association between the development of cardiovascular toxicity and an increased composite cardiovascular risk combining hypertension, stroke, coronary artery disease, atrial fibrillation, and heart failure (p=0.30). There was also no association between cardiovascular toxicity and a higher lifetime anthracycline dose (p=0.99), the type of CAR T cell therapy (p=0.073) or the presenting cancer type (p=0.65).
Conclusions:
Following CAR T cell therapy, cardiovascular adverse events are common and occur in association with CRS. These data support the need for a comprehensive pre-treatment cardiac evaluation and a cardiovascular surveillance strategy post-CAR T cell therapy.
Nielan:Parexel Imaging: Consultancy; BMS: Consultancy; AbbVie: Consultancy; Intrinsic Imaging: Consultancy; H3 Biomedicine: Consultancy. Yang:CSL Behring: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal